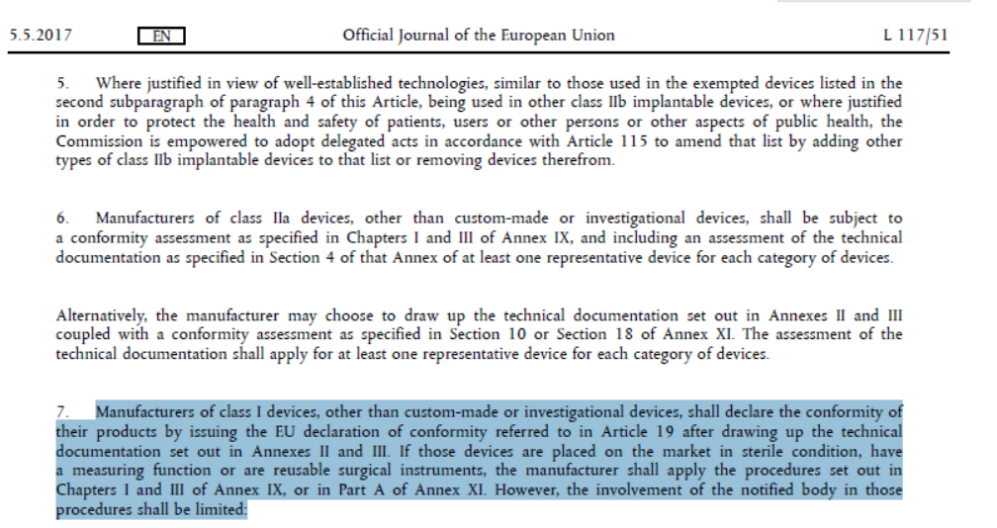
根据法规要求,普通一类(非灭菌,不带测量功能,非可重复使用外科类)医疗器械不需要认证机构进行审核,故没有CE证书。
在欧盟医疗器械法规MDR(2017/745) 52条第7节有明确说明,I类无菌或带有测量功能或重复使用的产品必须公告机构介入获取CE证书。而普通I类产品(比如:非无菌口罩和隔离衣)只需要按照MDR(7045/745)附录II和附录III准备CE技术文档,并进行自我符合声明就可以标识CE并出口欧盟市场。
具体法规截图如下: